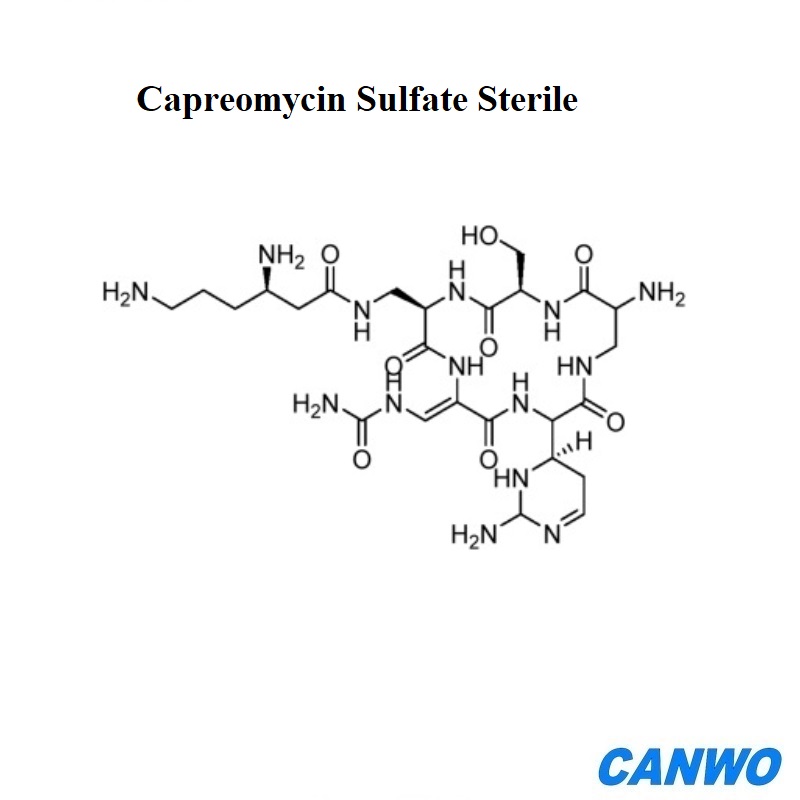
Capreomycin Sulfate Sterile
Standard: BP/USP/WHO/IN HOUSE
Ceftification: CHINA GMP/ WC / US FDA/WHO
MOQ: 1 KG
Price: Please send enquiry
Capreomycin Sulfate is a polypeptide antibiotic isolated from Streptomyces capreolus. Capreomycin Sulfate Sterile is high quality API without viable microorganisms produced under sterile pharmaceutical manufacturing processes. We supply Capreomycin Sulfate Sterile GMP standard with document support.
-
Standard: USP/ CP/ WHO
-
Certification: CHINA GMP/ WC / US FDA/WHO
-
Regulatory documentation: DMF open part, Letter of Access.
Indication: Capreomycin Sulfate is mainly used to treat tuberculosis (TB) together with other medicines.
Applications: Capreomycin Sulfate Sterile grade API can be used to produce Capreomycin Sulfate for injection( Capreomycin for Injection ).
Capreomycin Sulfate Sterile Detail Information
-
Molecular Formula:C25H46N14O11S
-
Molecular Weight:750.7849
-
CAS Registry Number:1405-37-4
-
Appearance: Capreomycin Sulfate API is white to practically white, amorphous powder. Freely soluble in water; practically insoluble in most organic solvents.
-
Packaging and storage:Preserve in tight, light-resistant containers.
-
Shelf life: 3 Years
-
Transport suggestion: Not Regulated.Capreomycin Sulfate Sterile raw material is not restricted as per special provision A3.