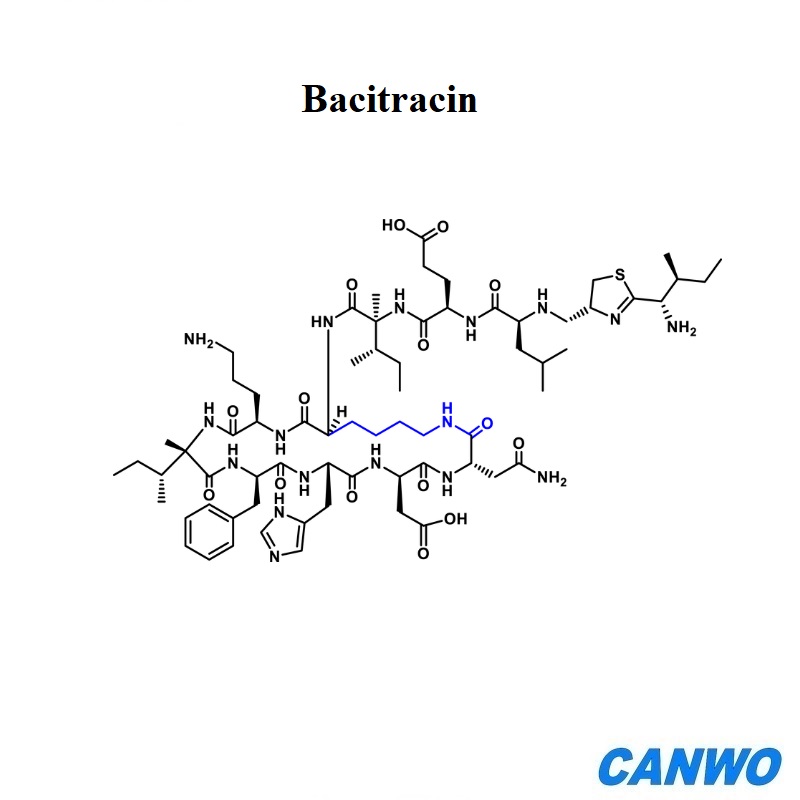
Cyclosporine
Standard: USP/ CP/ IN HOUSE
Packing: 1 kgs / tin, 1 kgs / bag or as requested
MOQ: 1 kgs
Price: Please send enquiry
Cyclosporine is a medicine called immunosuppressive agents that suppresses the immune system to prevent organ rejection or treat autoimmune diseases. We supply pharmaceutical raw material Cyclosporine GMP standard with document support.
-
Standard: USP/ CP/ IN HOUSE
-
Certification: CHINA GMP/ WC / US FDA
-
Regulatory documentation: DMF open part, Letter of Access.
Indication: Cyclosporine is used to prevent transplant rejection, to treat severe active rheumatoid arthritis, and to treat severe plaque psoriasis etc.
Applications: Cyclosporine API we supplied can be used to produce different dosage form including Cyclosporine Oral Solution,Cyclosporine capsules, Cyclosporine injection, Cyclosporine eye drops
Cyclosporine Detail Information
-
Molecular Formula:C62H111N11O12
-
Molecular Weight:1202.6112
-
CAS Registry Number:79217-60-0;59865-13-3
-
Appearance: Cyclosporine raw material is a white or almost white powder15. It is practically insoluble in water but easily soluble in ethanol and methylene chloride. Cyclosporine API contains not less than 97.0 percent and not more than 101.5 percent of cyclosporine A (C62H111N11O12), calculated on the dried basis.
-
Packaging and storage:Preserve in tight, light-resistant containers.
-
Shelf life: 3 Years
-
Transport suggestion: Not Regulated. Cyclosporine raw material is not restricted as per special provision A3.